Sustainable Solution-Safe Disposal of Arsenic Laden Waste
Of all the naturally occurring groundwater contaminants, arsenic is by far the most toxic, and its removal, therefore, must address the consequent disposal and/or containment issues. It was
recognized that coordinating collection and safe disposal of arsenic-laden sludge from individual households poses a level of complexity and enforcement effort that are difficult to sustain in remote villages. Installation of community-based arsenic removal units along with central regeneration facility offers a sustainable solution for arsenic removal and safe disposal of arsenic waste.
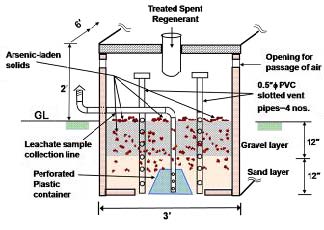
In regeneration process highly concentrated arsenic solution realized as spent regenerant is further treated with FeCl3 which prompt precipitation of arsenic along with ferric hydroxide. The entire amount of arsenic in spent regenerant is essentially transferred into the solid phase with ferric hydroxide precipitate, thereby leaving the rest of the solution essentially environmentally benign. The regeneration step allows reuse of the adsorbent media and reduces the volume of arsenic-laden sludge significantly. In contrast, nonregenerable adsorbent media are used universally for arsenic removal in the developed western world. After one-cycle application, such high-volume adsorbent media are routinely disposed of in landfills and hazardous waste sites. The treatment residuals in the form of sludge are contained in a coarse sand filter which is kept open to the atmosphere. A schematic of the coarse sand filter is shown in the following figure.
The composite pe-pH diagram in Figure 1a highlights three separate pre-dominance zones namely, neutralized regenerant waste, groundwater and landfill leachate. Clearly, Fe(III) and As(V) are pre-dominant in the aerated (oxidizing environment) neutralized regenerated sludge. Figure 1b displays the samples of arsenic laden sludge collected from the top of coarse sand filter in West Bengal, India. Figure 1c shows the laboratory simulated leaching test results of one sludge sample from West Bengal at different pH; under atmospheric conditions, both iron and arsenic leaching are relatively insignificant.

Figure 1. (a) composite arsenic-iron pe-pH diagram with groundwater, sludge and leachate predominance zone. (b) resultant sludge sample (c) Iron and arsenic leaching test result.
The pe-pH diagram (Figure 1a) also illustrates problems with arsenic disposal into traditional landfills; upon maturation of the landfill, microbial activity may influence the redox conditions, instigating arsenic mobility and leaching into groundwater systems. Both pH and redox condition uniquely determine speciation of arsenic and iron that in turn control arsenic leachability. Therefore the current disposal technique is scientifically more appropriate than dumping arsenic loaded adsorbents in the reducing environment of landfills as currently practiced in developed countries including USA.
