Why Does the Well-Head Unit Work So Well?
Since 1997, Bengal Engineering College in Howrah, India installed more than 175 well-head arsenic removal units in remote villages, several of them in areas bordering Bangladesh and India. Barring some minor perturbations, every unit has been consistently producing water with arsenic concentration well below 50µg/L, the maximum permissible arsenic concentration in drinking water in India and Bangladesh. The following figure presents arsenic concentrations in both influent and treated waters plotted against the number of bed volumes of water treated. Regardless of wide fluctuation in influent arsenic concentrations, arsenic remained well below 50 m g/L in the treated water for well over a year.
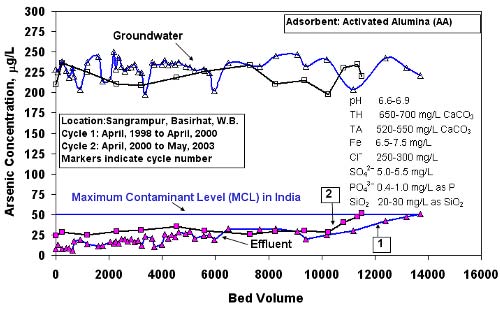
Arsenic concentration in treated and influent waters versus bed volumes of water treated in Sangrampur village, Basirhat, North 24 parganas, West Bengal
Both As(III) and As(V) species are significantly present in the contaminated groundwater; As(III) constitutes about 25-50% of the total dissolved arsenic. At circum-neutral pH of groundwater, As(V) exists as H2AsO4- and HAsO42-, while As(III) exists as nonionized H3AsO3. It is well known that activated alumina is ineffective in removing As(III) species. That is why such an excellent removal of total arsenic in well-head units for a prolonged time period (i.e., months) is counterintuitive and demands a scientific explanation.
Due to iron-rich soil in this region, the groundwater invariably contains a fairly high concentration of dissolved iron or Fe(II) ranging often from 2-10 mg/L. The top part of the well-head treatment unit is designed with large void space and a vent open to the atmosphere. As the hand pump is operated manually, the water entering the top part of the column is oxygenated, triggering oxidation of dissolved Fe(II) and subsequent precipitation of Hydrated Fe(III) Oxides (HFO). Oxidation of As(III) to As(V) oxyanion is, however, minimal at near-neutral pH due to much slower kinetics. The following figure shows photographs of fresh activated alumina particles (all white) prior to start-up of the well-head unit and the same particles with iron oxide precipitates (all brown) after a few days operation.

Oxidation of dissolved Fe(II) to insoluble Fe(III) hydroxide at near-neutral pH is a thermodynamically favorable process. At near-neutral pH in freshly precipitated hydrated Fe(III) oxide (HFO) particles, FeOH2+ and FeOH are pre-dominant functionalities which selectively bind both arsenates and arsenites oxyanions through formation of bidentate and/or monodentate inner-sphere complexes (Lewis acid-base interactions). As opposed commonly occurring anions (e.g chloride, sulfate, bicarbonate) usually present in relatively high concentration are weak ligands and show poor sorption affinity to HFO.
HAIX is essentially polymeric anion exchanger within which HFO nano-particles are irreversibly dispersed. It is scientifically proven that ligand sorption capacity of HFO nanoparticles within polymeric anion exchangers is greatly enhanced due to the Donnan membrane effect exerted by the fixed positive charges of anion exchangers. The following figure shows parent anion exchanger and hybrid anion exchanger (HAIX) loaded with HFO nano-particles.

The top part of the gravity-flow well-head column is deliberately designed with a large void space and a vent open to the atmosphere. As the hand-pump is operated manually, the groundwater entering the column first forms small droplets (i.e., larger surface area per unit volume) aided by a splash plate. The droplets subsequently get oxygenated, thus bringing the reaction of oxidation of Fe(II) to Fe(III) to near completion. The top chamber is followed by a regenerable sorbent material, spherical activated alumina and/or arsenic-selective hybrid anion exchanger (HAIX). The following figure shows (A) photograph of an actual arsenic removal unit and (B) salient reactions occurring at different stages of the unit.
Upon installation of an arsenic removal unit, a villager’s committee is set up to maintain daily backwashing operation and periodical monitoring of arsenic concentrations. Incorporation of such units into the socio-economic system of the village through community participation best ensures continued use, good water quality and proper waste disposal. Economic sustenance comes through realization of water tariff per month which is less than 50 US Cents per family. Depending upon arsenic concentration and other quality parameters of ground water, the units typically run for about 10,000 bed volumes (10 million liters) in each cycle which translates into one year of operation for supplying safe water for drinking and cooking for about 250 families. Once the effluent total As concentration reaches a cut-off limit of 50µg/L, the adsorbent media is removed and shipped to the central regeneration facility at Maslandpur in N. 24 Parganas, West Bengal, India.
